Research
Our research currently focuses on: (1) understanding the molecular pathogenesis of B cell lymphomas, particularly that of diffuse large B cell lymphoma (DLBCL), with use of patient tumor materials and genetic mouse models; (2) further elucidating a newly discovered immune surveillance mechanism on Epstein-Barr virus (EBV)-infected/transformed B cells. We then leverage the resulting knowledge to develop new approaches for cancer therapy.
Genetically engineered mice are crucial to our research. They allow us to interrogate the roles of specific genetic events in tumor initiation and progression, and to reveal the responses of immune cells during these processes in a dynamic manner.
DLBCL, the most common type of B cell lymphoma in adults, is a genetically, phenotypically, and clinically heterogeneous disease. Gene expression profile analysis revealed several subtypes, including germinal center B cell (GCB)-like and activated B cell (ABC)-like DLBCL. The less curable ABC-DLBCL often carries genetic lesions leading to constitutive NF-kB activation and interference with terminal B cell differentiation; the latter lesions include deletion/mutation of BLIMP1 and translocation of BCL6. Using conditional gain- and/or loss-of-function mutagenesis in mice, we have demonstrated the cooperation of the canonical NF-kB signaling and disruption of BLIMP1 in the pathogenesis of ABC-DLBCL. Mechanistically, the canonical NF-kB signaling enhances B cell proliferation and survival, but also terminal differentiation; the latter process is abolished upon BLIMP1 loss (see Calado and Zhang et al. Cancer Cell 2010). Furthermore, we revealed the synergy of the alternative (non-canonical) NF-kB signaling with deregulation of BCL6 in DLBCL pathogenesis in a sizable fraction of cases (see Zhang et al. Cell Reports 2015). Our ongoing efforts are unraveling the roles of other genes, including the dual-specificity phosphatase 2 (DUSP2), in B cell lymphomagenesis.
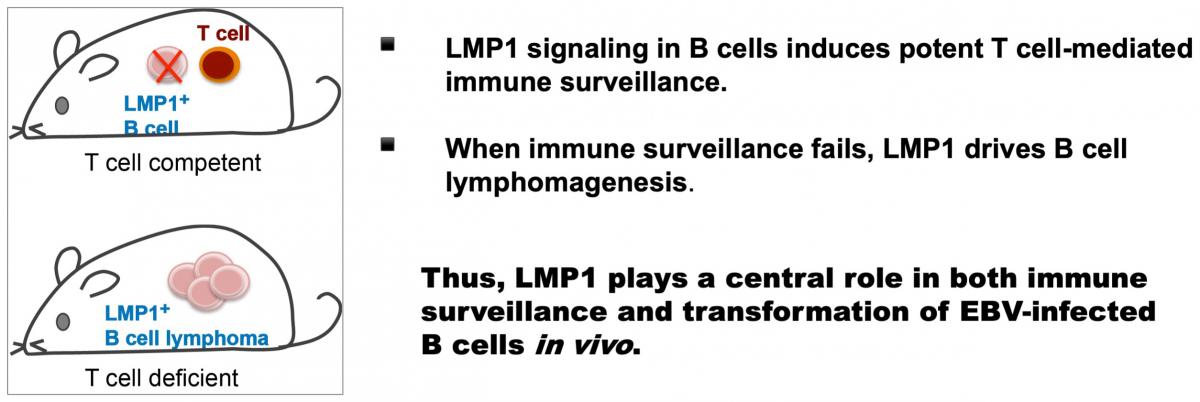
EBV is a herpes virus that specifically infects, and can transform, human B cells. More than 90% of the human population is EBV-infected. The infected B cells are rapidly cleared by the immune system, but in a minute fraction of B cells the virus acquires a latent state and persists for life. When immune surveillance fails, fatal lymphoproliferation and lymphomagenesis ensue. We have generated mice expressing the EBV signaling protein LMP1 in B cells in a constitutive or inducible manner. Our studies in these mouse models revealed a central role for LMP1 in both immune surveillance and transformation of EBV-infected B cells in vivo: expression of LMP1 in B cells induces potent T cell responses which in turn eliminate LMP1+ B cells; upon T cell depletion, LMP1 drives rapid B cell proliferation and lymphomagenesis (see Zhang et al. Cell 2012).
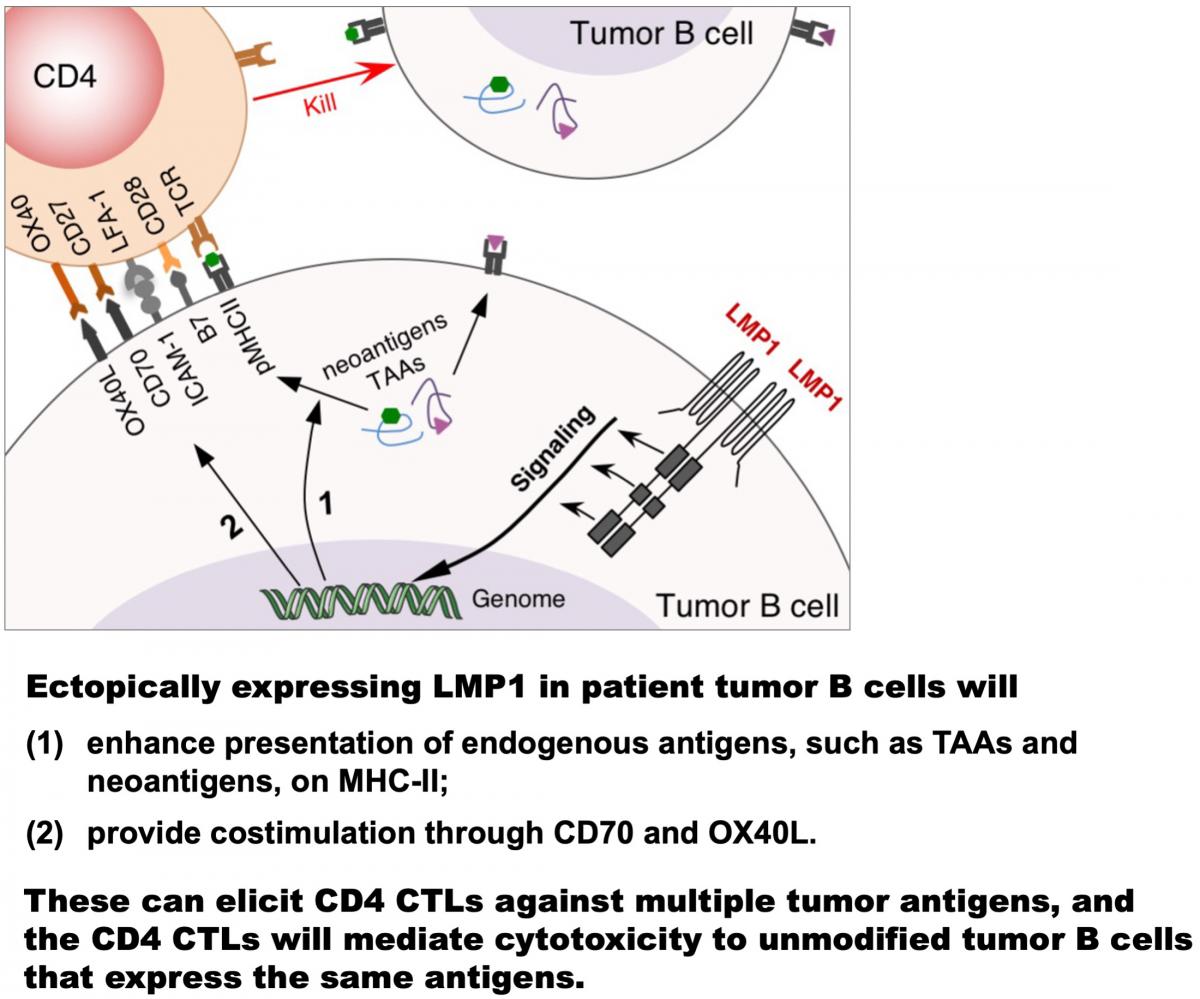
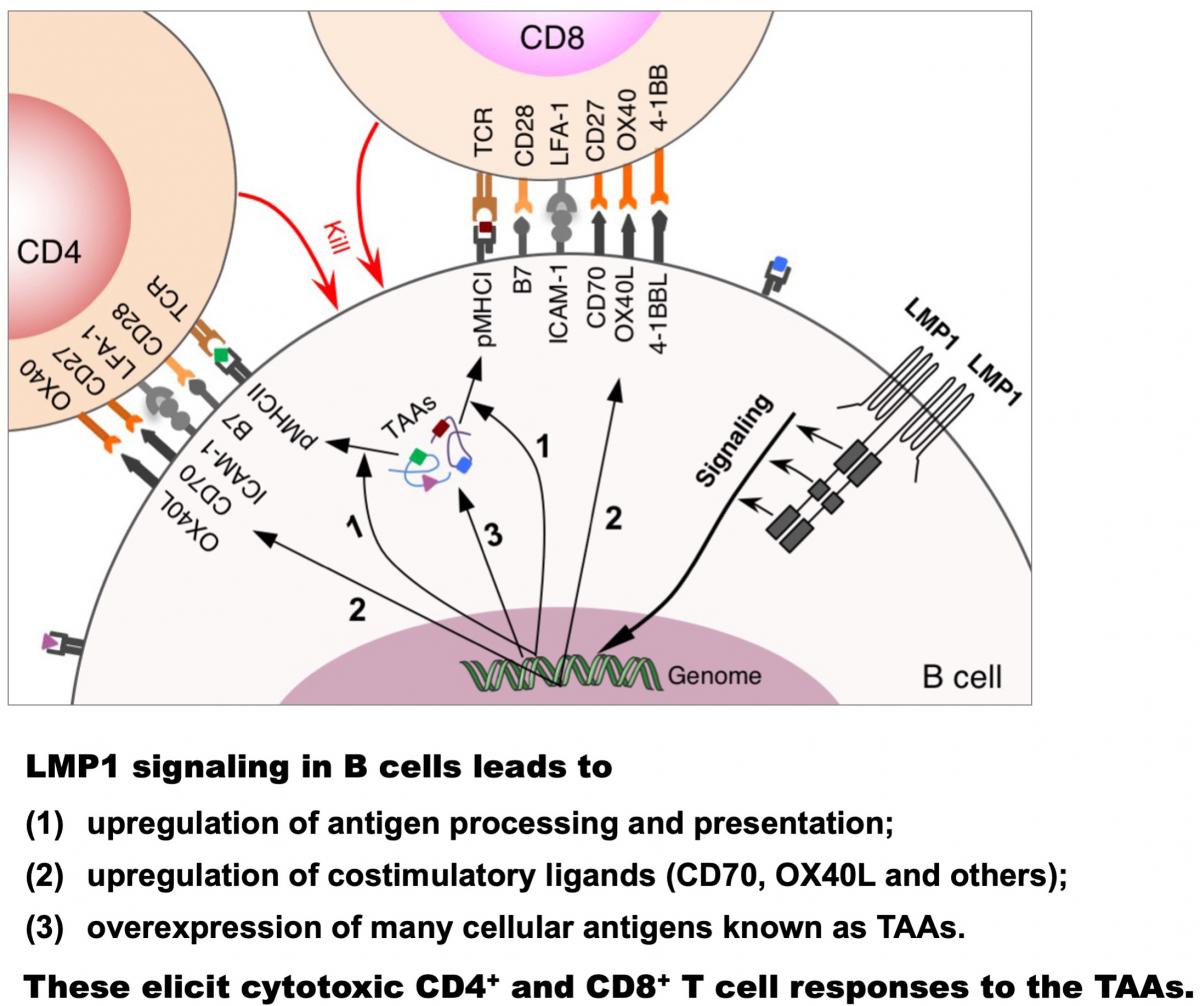
Further studies showed that LMP1 signaling in B cells enhances presentation of endogenously expressed antigens, including a wide range of LMP1-induced cellular antigens known to function as tumor-associated antigens (TAAs), on MHC classes I and II, and simultaneously provides costimulation through CD70 and OX40 ligand (OX40L), thereby eliciting cytotoxic CD4+ and CD8+ T cell responses against these TAAs (see Choi et al. PNAS 2018 and Nature 2020). These findings thus delineate a mechanism of infection-induced anti-tumor immunity, making conceptual changes in understanding viral and tumor immunity. An ongoing project is examining the impact of EBV-induced TAA-specific T cell immunity on human cancers.
Exploiting LMP1, we have developed a novel approach to produce multi-specific cytotoxic CD4+ T cells (CD4 CTLs) for treating B cell malignancies. This enables targeting a wide range of endogenous tumor antigens, such as TAAs and neoantigens, without the need to identify them in each patient (see Choi et al. Nature 2020). Efforts to translate the CD4 CTL-based adoptive cell therapy to clinical testing as a standalone therapy and in combination with immune-checkpoint blockade are underway. Meanwhile, we endeavor to develop LMP1-based immune approaches for other cancers.